-Advertisement-
-Advertisement-
Rheumatology
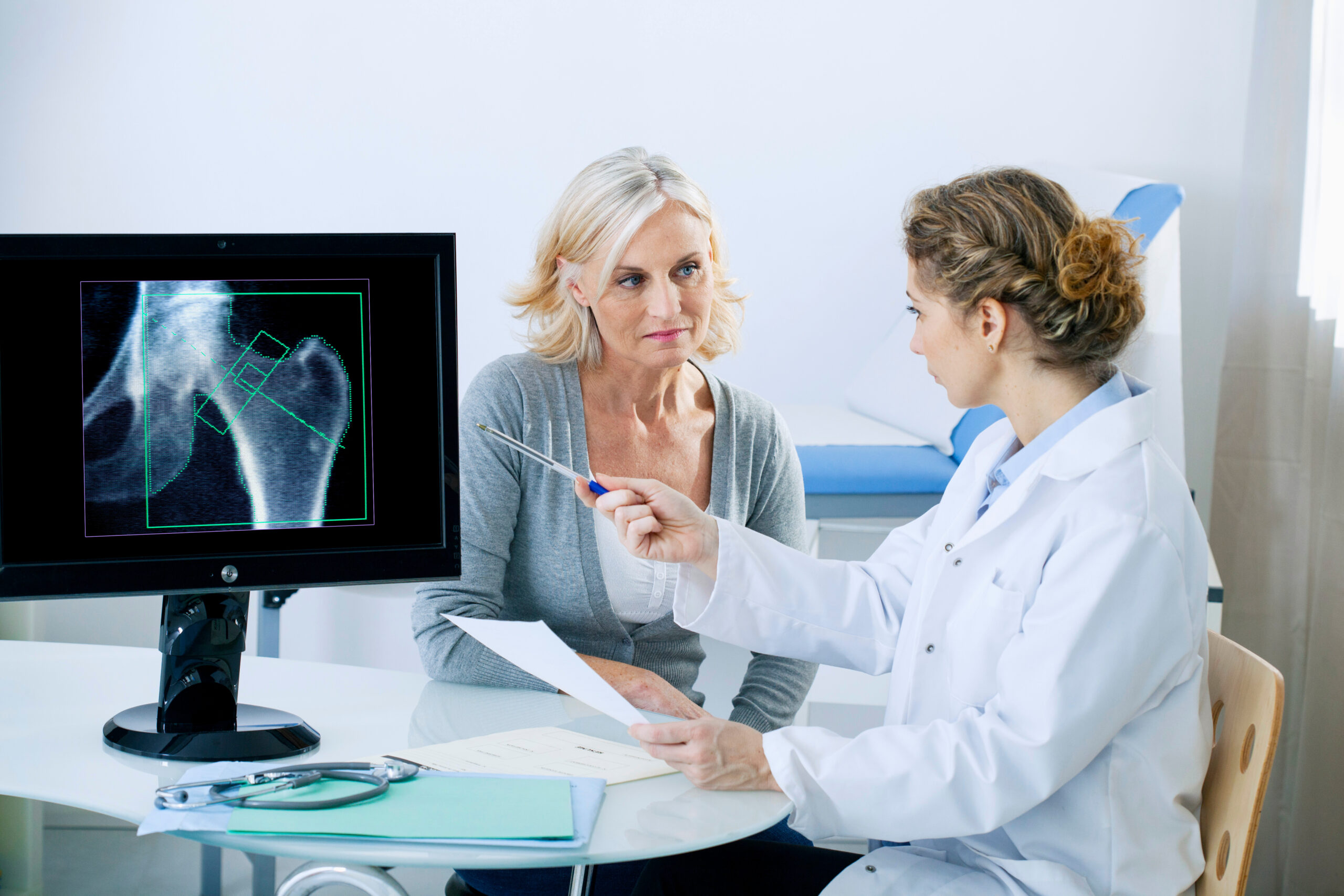
FDA approves Kevzara for treatment of active polyarticular juvenile idiopathic arthritis
The U.S. Food and Drug Administration has approved Kevzara (sarilumab) for the treatment of patients weighing 63 kg or greater with active polyarticular juvenile idiopathic arthritis (pJIA). This form of arthritis affects multiple joints simultaneously and can significantly impact the lives of affected children. In clinical trials, the pJIA population...
Read More-Advertisement-
-Advertisement-
-Advertisement=-
-Advertisement-
-Advertisement-
-Advertisement=-
-Advertisement-

Contact
© 2024 Med Journal 360™ is a trademark of International Healthcare Media, LLC. All rights Reserved